
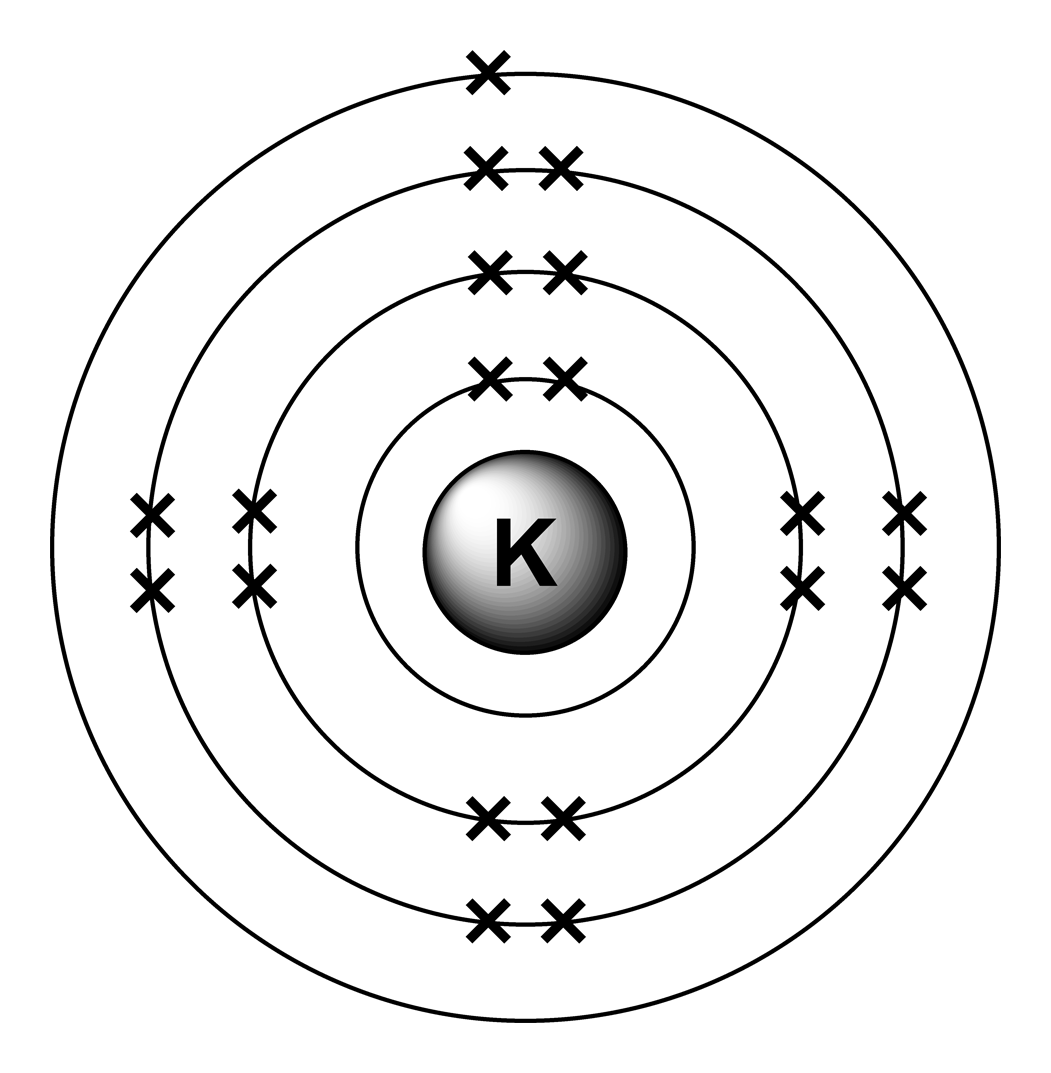
Have you ever heard about getting a shock from a socket, static electricity, or lightning? Those are all related to electric charges. You can see that each part of the atom is labeled with a "+", "-", or a "0." Those symbols refer to the charge of the particle. The atomic number is also called the proton number. That number tells you the number of protons in every atom of the element. Every element is unique and has an atomic number. The atoms of different elements have different numbers of electrons, protons, and neutrons. (117 as we write this) Chemists and physicists are trying to make new ones every day in their labs. There are almost 120 known elements in the periodic table. They group together in the center of the atom. Protons and neutrons are found in the nucleus. Electrons are found in shells or orbitals that surround the nucleus of an atom. What are electrons, protons, and neutrons? Electrons are the smallest of the three particles that make up atoms. However, science is based on the atom because it is the smallest distinct unit of matter.Įven though many super-tiny atomic particles exist, you only need to remember the three basic parts of an atom: electrons, protons, and neutrons.
Nuclear chemists and physicists work together at particle accelerators to discover the presence of these tiny, tiny, tiny pieces of matter. These subatomic particles include nucleons and quarks. Super-small particles can be found inside the pieces of atoms. As you learn more, you can move to the reactions and biochemistry pages and see how atoms form compounds that help the biological world survive.Īre there pieces of matter that are smaller than atoms? Sure there are. We're going to cover basics like atomic structure and bonding between atoms. Solids are made of densely packed atoms while gases have atoms that are spread out.
/atomic-structure-artwork-549603139-57fe40e75f9b586c3537ebf4.jpg)
As you know, matter is composed of atoms.
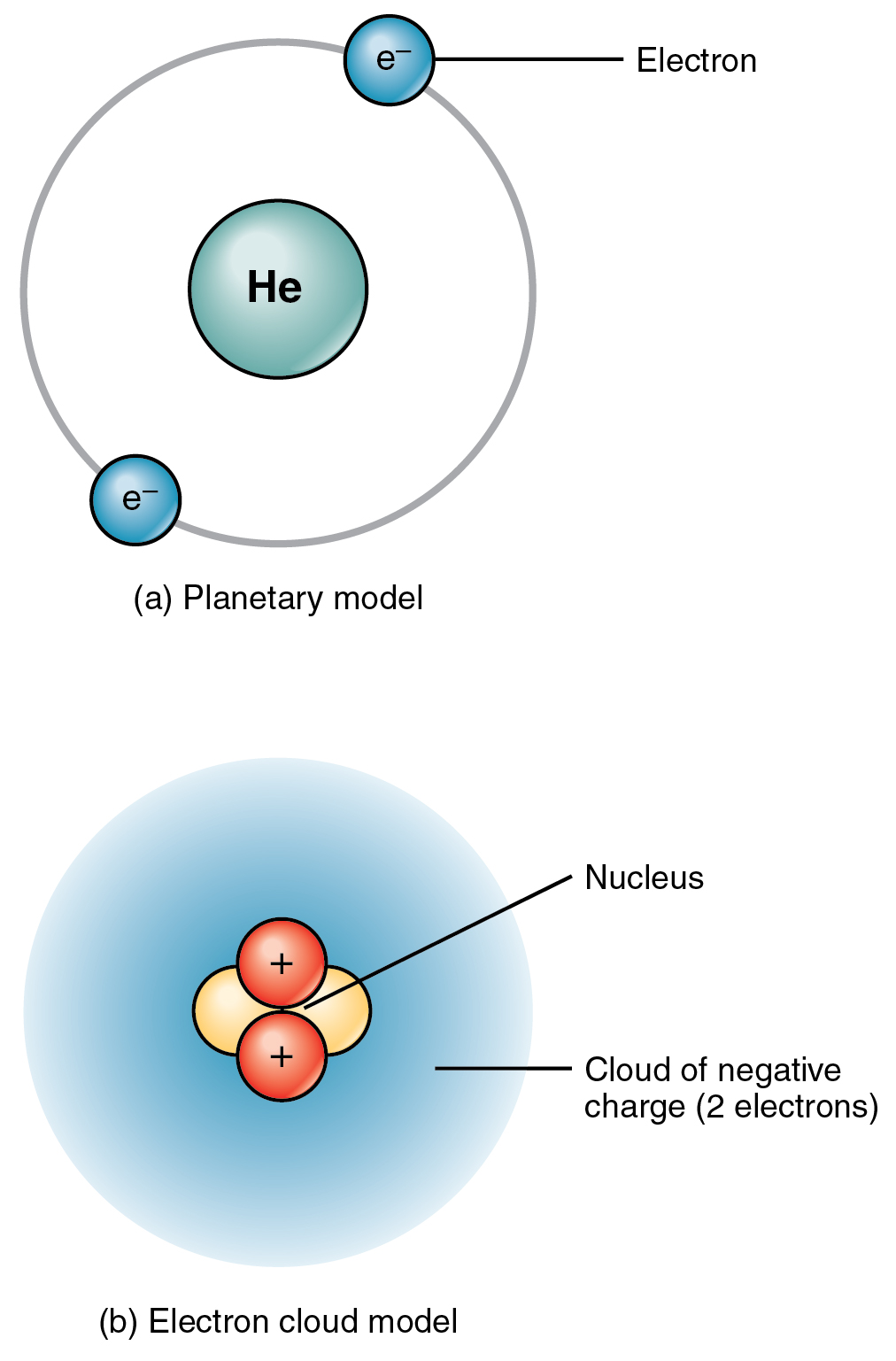
They are the basis for everything in the Universe.


 0 kommentar(er)
0 kommentar(er)
